Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 7 surface observed by synchrotron radiation real-time photoelectron spectroscopy
7 surface observed by synchrotron radiation real-time photoelectron spectroscopyYoshigoe, Akitaka; Teraoka, Yuden
no journal, ,
Recent experimental works regarding the metastable oxygen adsorbate on Si(111)-7 7 at room temperature report that the oxygen molecule chemisorbs on the Si adatom inherently having some oxygen atoms at the backbond sites. Since the numbers of oxygen atoms at the backbond sites of the Si adatom have been ambiguous only from O1s XPS, the mechanism of initial oxidation processes has been under debate. In this talk, we report the initial oxygen adsorption processes investigated by real-time Si2p XPS using high resolution synchrotron radiation in addition to O1s one. All experiments were performed at SUREAC2000 at BL23SU in SPring-8. The oxygen gas at 5.3
7 at room temperature report that the oxygen molecule chemisorbs on the Si adatom inherently having some oxygen atoms at the backbond sites. Since the numbers of oxygen atoms at the backbond sites of the Si adatom have been ambiguous only from O1s XPS, the mechanism of initial oxidation processes has been under debate. In this talk, we report the initial oxygen adsorption processes investigated by real-time Si2p XPS using high resolution synchrotron radiation in addition to O1s one. All experiments were performed at SUREAC2000 at BL23SU in SPring-8. The oxygen gas at 5.3 10
10 Pa was exposed to the clean Si(111)-7
Pa was exposed to the clean Si(111)-7 7 surface at room temperature and the oxidation process was in-situ monitored by real-time XPS for O1s and Si2p alternate measurement using synchrotron radiation. Comparing the chemical shifts of O1s with those of Si2p XPS, we found that the oxidation states corresponding to Si
7 surface at room temperature and the oxidation process was in-situ monitored by real-time XPS for O1s and Si2p alternate measurement using synchrotron radiation. Comparing the chemical shifts of O1s with those of Si2p XPS, we found that the oxidation states corresponding to Si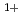 and Si
and Si were formed at the observable dosage for the metastable O
were formed at the observable dosage for the metastable O adsorbate. Thus, it is found that the metastable O
adsorbate. Thus, it is found that the metastable O species adsorb at the on-top site of Si adatom bonding to an oxygen atom at that backbond, i.e. ins-paul structure. Moreover, the oxidation states with higher numbers, such as Si
species adsorb at the on-top site of Si adatom bonding to an oxygen atom at that backbond, i.e. ins-paul structure. Moreover, the oxidation states with higher numbers, such as Si and Si
and Si , appeared at the oxidation time when the metastable oxygen adsorbate almost vanished. This result suggests that the oxygen molecule of an ins-paul dissociates to create two oxygen atoms. One oxygen atom remains at the on-top site of the Si adatom and it is assigned to be an ad-oxygen. The other moves to the Si adatom backbond sites to become an ins-oxygen. We concluded that the Si oxidation structure is the insx2-ad structure just after the disappearance of metastable oxygen adsorbate.
, appeared at the oxidation time when the metastable oxygen adsorbate almost vanished. This result suggests that the oxygen molecule of an ins-paul dissociates to create two oxygen atoms. One oxygen atom remains at the on-top site of the Si adatom and it is assigned to be an ad-oxygen. The other moves to the Si adatom backbond sites to become an ins-oxygen. We concluded that the Si oxidation structure is the insx2-ad structure just after the disappearance of metastable oxygen adsorbate.
 2 surface
2 surfaceSuemitsu, Maki*; Togashi, Hideaki*; Takahashi, Yuya*; Yamamoto, Yoshihisa*; Teraoka, Yuden; Yoshigoe, Akitaka; Asaoka, Hidehito
no journal, ,
Si(110) surface has attracted much attention for its use in the next-generation high-speed and highly-integrated devices. We have investigated the effect of mild annealing on the RT-oxidized Si(110)-16 2 surface by using SR-XPS and STM. After an annealing at 573K for 15min, the Si2p spectrum presented a decrease in the relative intensities of Si
2 surface by using SR-XPS and STM. After an annealing at 573K for 15min, the Si2p spectrum presented a decrease in the relative intensities of Si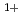 and Si
and Si suboxides and an increase in those of Si
suboxides and an increase in those of Si and Si
and Si suboxides, indicating clustering of oxygen atoms. The STM image from the annealed surface actually presented a clustered structure named "DD site", at which clustered Si adatoms appear dark for both the filled and the empty states. As far as the oxidation of Si(110) clean surface is concerned, it was clarified that there is an oxidized state that is observed completely dark by STM.
suboxides, indicating clustering of oxygen atoms. The STM image from the annealed surface actually presented a clustered structure named "DD site", at which clustered Si adatoms appear dark for both the filled and the empty states. As far as the oxidation of Si(110) clean surface is concerned, it was clarified that there is an oxidized state that is observed completely dark by STM.
 on Cu surfaces
on Cu surfacesMoritani, Kosuke*; Tsuda, Muneyuki*; Teraoka, Yuden; Okada, Michio*; Yoshigoe, Akitaka; Fukuyama, Tetsuya*; Kasai, Toshio*; Kasai, Hideaki*
no journal, ,
Oxygen molecules adsorbe dissociatively at Cu(111), Cu(110), and Cu(001) surfaces. The oxygen uptake curves were investigated by using supersonic molecular beam techniques and photoemission spectroscopy with synchrotron radiation. Adsorption probability increased with increasing translational kinetic energy of oxygen molecules. Vibrational and rotational levels of oxygen molecules were excited by elevation of nozzle temperature up to 1400 K keeping the incident energy of 0.5 eV. Although the adsorption probability increased with increasing nozzle temperature by 1000 K, it decreased inversely around 1400 K. These phenomena were interpreted as adsorption probability increase by vibrational excitation and decrease by rotational excitation.
 adsorption at Cu and Cu
adsorption at Cu and Cu Au
AuOkada, Michio*; Moritani, Kosuke*; Teraoka, Yuden; Yoshigoe, Akitaka; Kasai, Toshio*
no journal, ,
Oxidation processes at Cu surfaces induced by supersonic oxygen molecular beams has been studied by photoemission spectroscopy with synchrotron radiation. Oxidation processes of Cu(001), Cu(110), Cu(111), and Cu(410) has been investigated by now. Following conclusions were obtained. Cu O structures were formed even at room temperature by an action of translational kinetic energy of oxygen molecules. The formation of Cu
O structures were formed even at room temperature by an action of translational kinetic energy of oxygen molecules. The formation of Cu O structure is due to adsorbed-oxygen absorption mechanisms induced by succesive molecular collisions. Furthermore, Cu oxide layer is formed as the first layer and an Au underlayer as the second layer protects further oxidation.
O structure is due to adsorbed-oxygen absorption mechanisms induced by succesive molecular collisions. Furthermore, Cu oxide layer is formed as the first layer and an Au underlayer as the second layer protects further oxidation.
Takakuwa, Yuji*; Ogawa, Shuichi*; Ohira, Masayuki*; Ishizuka, Shinji*; Yoshigoe, Akitaka; Teraoka, Yuden; Mizuno, Yoshiyuki*; Yamauchi, Yasuhiro*; Homma, Teiichi*
no journal, ,
Oxidation processes of Ti(0001)-1 1 clean surface were analyzed by real-time monitoring using a variety of surface analysis methods of XPS, UPS, and AES/RHEED. Following conclusions were obtained by these observations. Oxide layer grows epitaxially at the Ti(0001) surface with a
1 clean surface were analyzed by real-time monitoring using a variety of surface analysis methods of XPS, UPS, and AES/RHEED. Following conclusions were obtained by these observations. Oxide layer grows epitaxially at the Ti(0001) surface with a 

 structure. Surface roughness changes periodically. The period is consistent with that of work function. Low oxidation state (TiO) plays a dominant role in the dissociative adsorption of oxygen molecules. The oxide layer decomposes easily at surface temperature over 673 K. The TiO
structure. Surface roughness changes periodically. The period is consistent with that of work function. Low oxidation state (TiO) plays a dominant role in the dissociative adsorption of oxygen molecules. The oxide layer decomposes easily at surface temperature over 673 K. The TiO structure reduces to TiO during thermal decomposition.
structure reduces to TiO during thermal decomposition.